Mucosal Barriers Were Made to Keep Things Out - Including Therapeutics
Local treatments exist but they’re often inefficient. Systemic treatments risk high off-target exposure to compensate for the poor uptake. The Result? More side effect, higher dosing burden and less relief for patients.
MUCOSAL DELIVERY TODAY IS OFTEN:
Frequent drops, sprays or suppositories
Poor absorption and short residence time
High dosing burden and poor adherence
MUCOSAL DELIVERY SHOULD BE INSTEAD:
Stay where it’s needed and act locally
Release the right dose at the right time
Be painless, invisible, and effortless
It doesn’t have to be this way. Our approach delivers drugs directly to mucosal surfaces and keeps them there without relying on high doses or systemic exposure.
Our Drops Can Do Better
20|20 OptimEyes has created a drop that can reduce dosing to once a week. While this drop can be used for many ocular conditions, we are currently validating it for glaucoma with a latanoprost formulation.
We have validated this technology through various in-vivo studies. After a single dose, our micelles were found at 6x greater quantities on the eye, showing our sustained effect. We have shown equivalent efficacy to Restasis in a dry eye rat model when dosing once every three days compared to twice daily as currently prescribed.
A preliminary pharmacokinetic study has shown our formulation has similar corneal drug levels to Restasis when dosed once every three days compared to Restasis twice daily. When our formulation is dosed twice daily, we show 3x greater drug levels in the tissue.
20|20 OptimEyes Technologies has developed a drug delivery MNP eye drop formulation that allows greater residence time on the eye. This patented platform technology:
Encapsulates drug in its core allowing greater drug loading
Binds to the ocular surface increasing its residence time per dose
Allows sustained release with greater bioavailability
Decreases ocular irritation using its aqueous formulation
When a single dose is given, the concentration of our OptimEyes MNPs on the ocular surface is 2x greater than a standard MNP after 48 hours. By 1 week, our concentration is 6x than a standard MNP, demonstrating the importance of the binding moiety on our MNPs.
MNPs Enable Key Differentiators
A Transformative Platform for Sustained Mucosal Drug Delivery
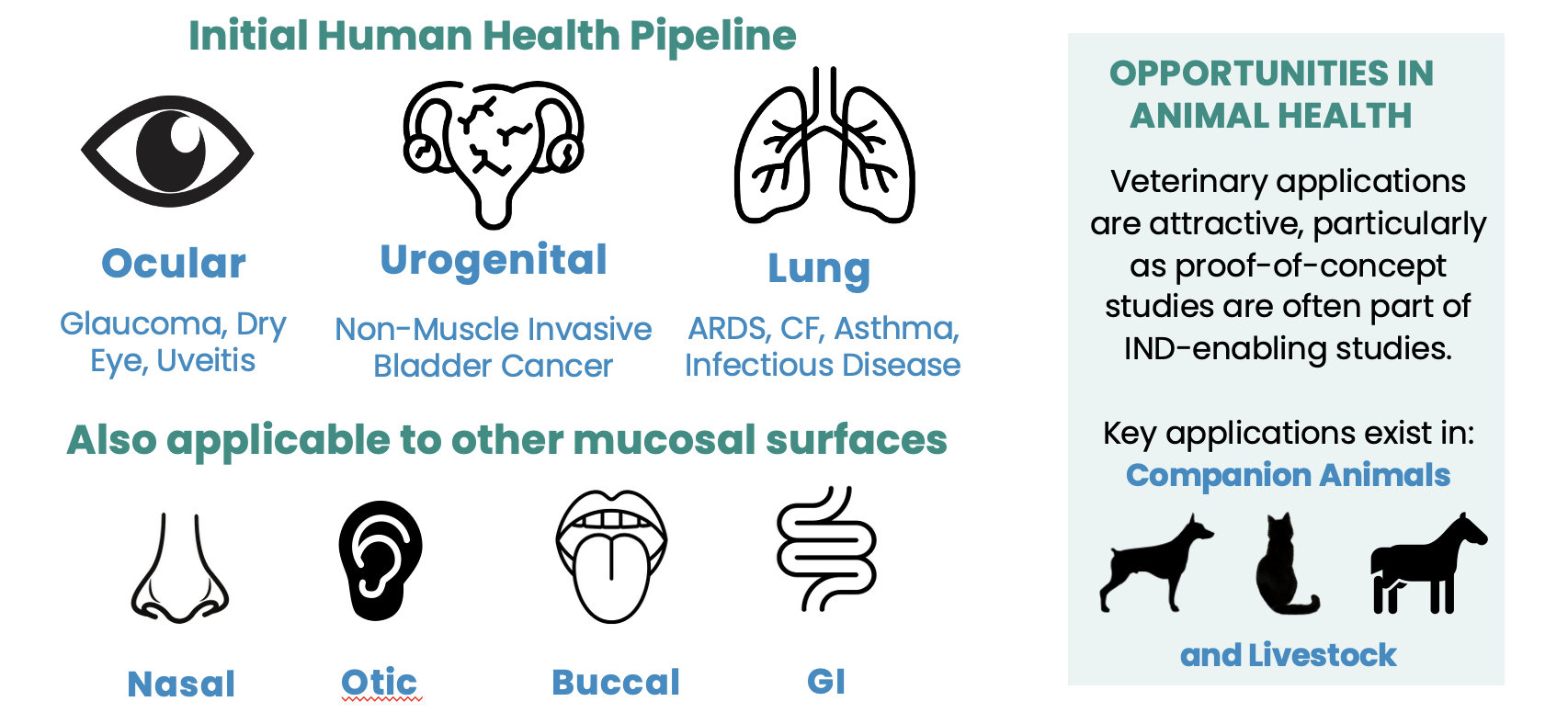
The MNP platform unlocks product opportunities in ophthalmology, respiratory, urogenital, gastrointestinal and CNS pathologies
Platform Expansion Opportunities Exist at Other Mucosal Surfaces
Beyond glaucoma, our platform enables delivery of anti-inflammatory, antiviral and other classes of therapeutics across all mucosal barriers for human and veterinary indications.
The United States (Patent No. 10611908), Europe (Patent No. EP3344232B1); Canada (Patent No. CA2996910C); and Japan (Patent No. JP2018529696A) patents for this technology have all been granted.